how do leucine zippers work
The leucine zippers may be homo- or heterodimeric and thus increase their specificity by a combinatorial control mechanism. Uploaded on Sep 25 2014.
The a positions targeted for mutagenesis are highlighted in boldface and are underlined.

. In the course of one such study the X-ray structure of the. In this work we have focused on the ribonuclease activity of the authentic leucine zippers of the bZIP regions of yeast GCN4 and human c-Jun. The leucine zipper is a dimerization domain occurring mostly in regulatory and thus in many oncogenic proteins.
BZIP proteins are transcription factors that consist of three modular functional regions mediating dimerization DNA binding and transcriptional regulation. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an α-helical conformation which facilitates. Transcription factors containing bZIP domains are found across eukaryotes from budding yeast to humans.
Gcn4 Basic Region Leucine Zipper Complex With Ap-1 DNA. The basic region of the bZIP domain rich in lysines. FRCPath 10 th December 2009.
The coiled coil structure of a leucine zipper is required for dimerization and can be predicted with reasonable accuracy. Basic-region leucine zipper bZIP proteins are one of the largest transcription factor families that regulate a wide range of cellular functions. C-Jun where the leucine zipper domain has become involved in transcription activation but this is the exception.
The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an α-helical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving. The hallmark of these proteins is the bZIP basic region leucine zipper domain a well-defined motif in eukaryotic proteins 1 2. Basic leucine zipper bZIP proteins are a class of transcription factors characterized by a basic leucine zipper motif which allows for both dimerization and sequence-specific DNA-binding interactions Ellenberger 1994.
The basic leucine zipper bZIP contains 25 conserved amino acids of these nine substitutions and disrupts function. The leucine zipper is a proteinprotein interaction domain consisting of amphipathic a helices that dimerize in parallel either as homodimers or heterodimers. Three families of leucine zipper proteins are known the basic region leucine zipper proteins bZip the basic region helix-loop-helix leucine zipper proteins bHLH-Zip homeobox DNA binding domain leucine zipper proteins.
B Side view of the dimerThe amino acid backbones in a helical conformation are represented. 1805 Views Download Presentation. Transcription factors containing leucine zipper or zinc finger motifs would thus combine transcription activation with slow RNA degradation.
The leucine repeat in the sequence has been traditionally used for identification however with poor reliability. Owing to the stability of their coiled coil structure leucine zipper LZ domains of bZIP factors are widely employed as dimerization motifs in protein engineering studies. We show that the activity of these leucine.
The leucine zipper of GCN4 arranged as a parallel coiled coil. A Sequence of the leucine zipper as it occurs in the λ repressor fusion systemLowercase letters indicate positions in the heptad repeat. There are a few cases eg.
My Masters Work -. Leucine Zipper domains allow subunits of a transcription factor to bind together. In some transcription factors the leucine zipper domain extend into a new sequence-specific DNA binding domain as well.
Leucine zipper domains are made up of two motifs. A basic region that recognizes a specific DNA sequence and a series of leucines spaced 7 residues apart along an α-helix leucine zipper that mediate dimerization. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an αhelical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving.
These motifs form a continuous α-helix that can dimerize through formation of a coiled-coil structure involving paired contacts. Work on the trimeric coiled-coil in the influenza haemag-glutinin receptor Carr and Kim 1993At physiological. Of active oxygen species is a type of stress called photo.
The binding domain is usually 100-amino-acid long. These can form homodimers and heterodimers through their leucine-zipper domains.

Helical Wheel Representation Of The Leucine Zipper Domain Of The Download Scientific Diagram

Leucine Zippers Leucine Zipper Of The Yeast Activator Protein Gcn4 Download Scientific Diagram

X Ray Structure Of Gcn4 B Zip Dimer Bound To Double Stranded Dna 10 Download Scientific Diagram

Amino Acid And Nucleotide Sequence Of The Fos Leucine Zipper A And Of Download Scientific Diagram

Dimerization Occurs Through An Unusual Leucine Zipper Motif A Helical Download Scientific Diagram
A Sequence Alignment Of Leucine Zippers Used In Experiments With Vbp Download Scientific Diagram

Leucine Zipper An Overview Sciencedirect Topics

Structure Based Design Of A Zinc Finger Leucine Zipper Dimer 76 A Download Scientific Diagram

Two Distinct Mechanisms Of Basic Leucine Zipper Containing Domain Download Scientific Diagram

Basic Leucine Zipper Transcription Factor An Overview Sciencedirect Topics

Attractive Interhelical Electrostatic Interactions In The Proline And Acidic Rich Region Par Leucine Zipper Subfamily Preclude Heterodimerization With Other Basic Leucine Zipper Subfamilies Journal Of Biological Chemistry

Dimerization Via Tandem Leucine Zippers Is Essential For The Activation Of The Mitogen Activated Protein Kinase Kinase Kinase Mlk 3 Journal Of Biological Chemistry
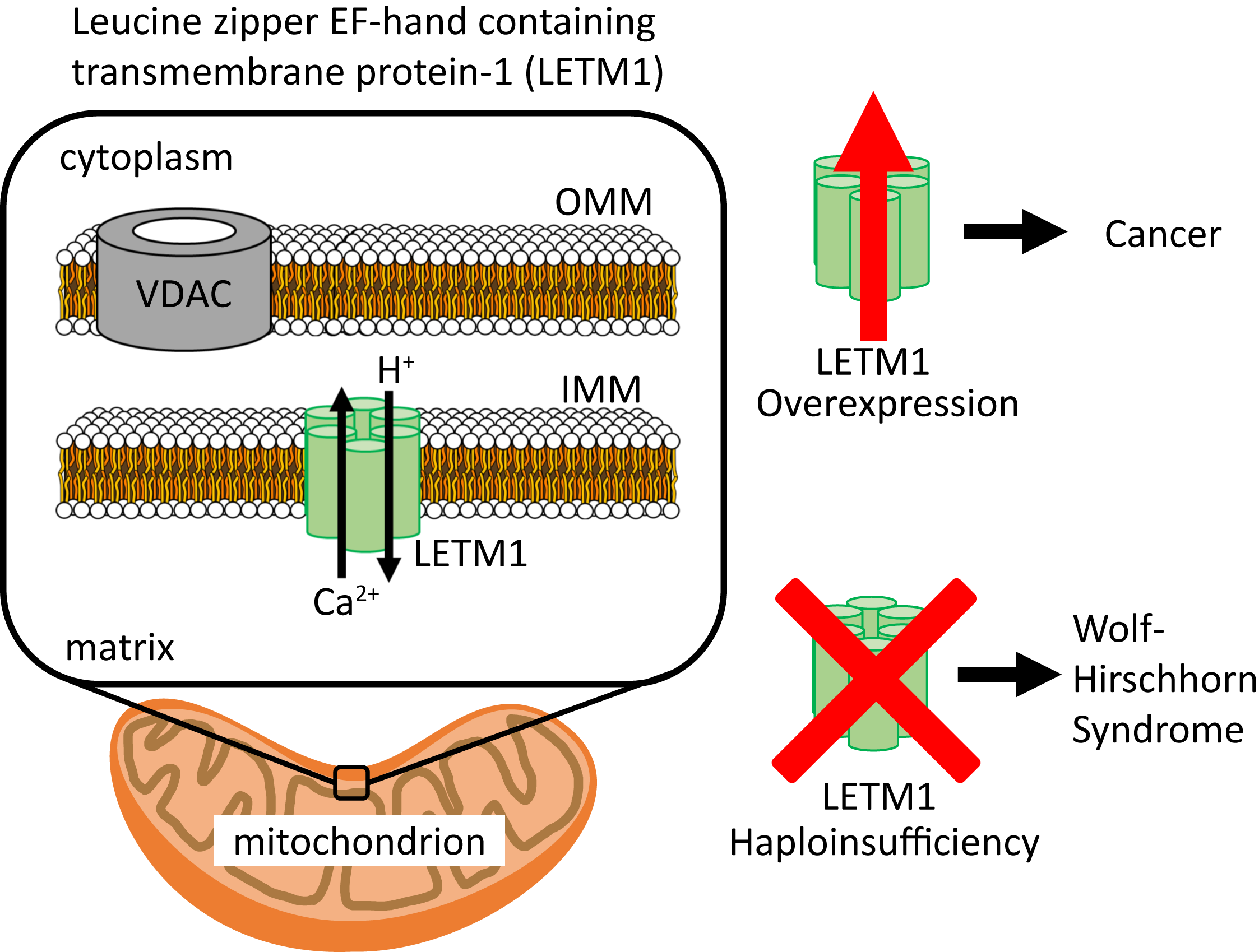
Ijms Free Full Text Molecular Mechanisms Of Leucine Zipper Ef Hand Containing Transmembrane Protein 1 Function In Health And Disease Html

Amino Acid Sequence Of 7 Human Leucine Zipper Regions Proteins Are Download Scientific Diagram

Fosb Jund Bound To Cognate Dna A Bzip Domain With Leucine Zipper And Download Scientific Diagram

Crystal Structure Of The Ccaat Box Enhancer Binding Protein B Activating Transcription Factor 4 Basic Leucine Zipper Heterodimer In The Absence Of Dna Journal Of Biological Chemistry

A Interactions Between Anti Parallel Leucine Zippers Lz Dashed Download Scientific Diagram

Leucine Zippers Leucine Zipper Of The Yeast Activator Protein Gcn4 Download Scientific Diagram

